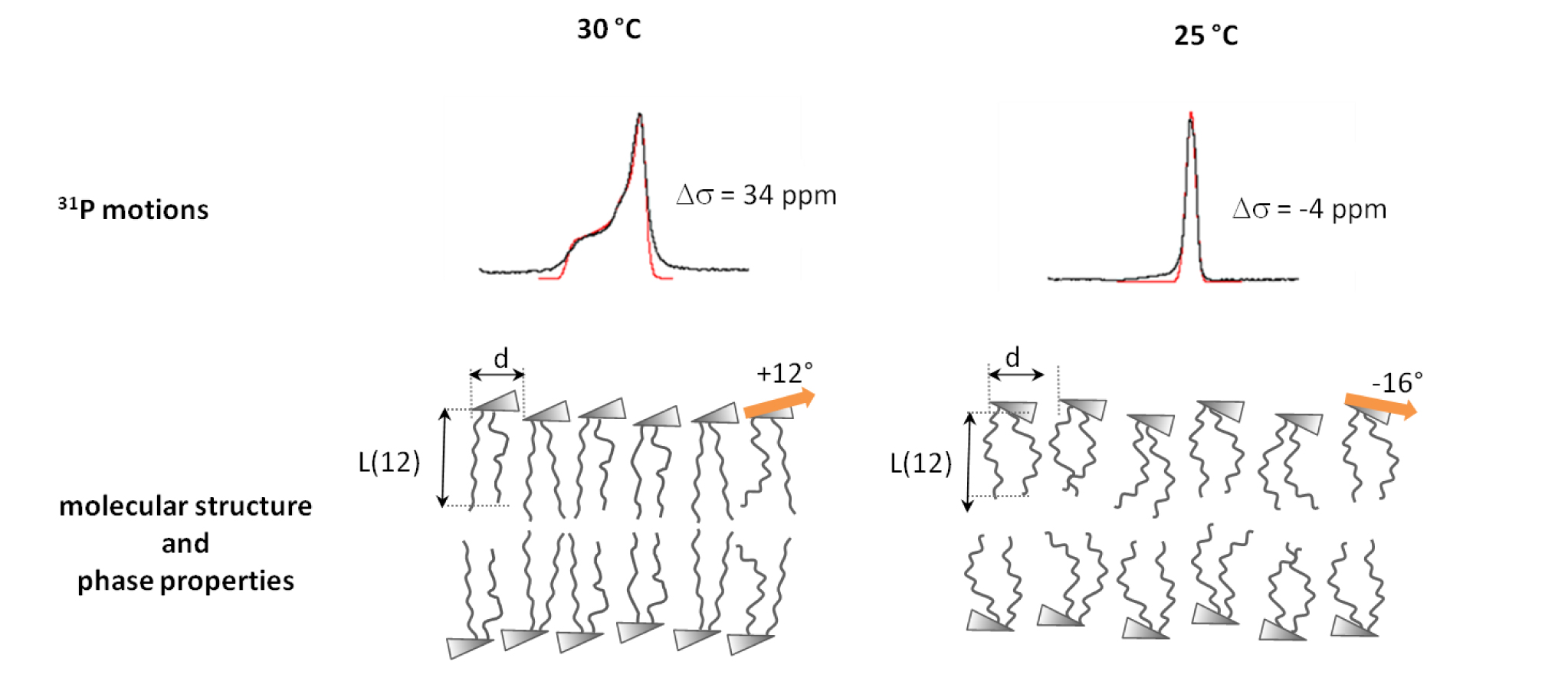
Cell penetrating peptides, Alzheimer peptides and membrane-induced protein folding
Cell penetrating peptides (CPP)
We could exclude the still popular model that the induction of nonbilayer structures plays a role in CPP membrane translocation. We observed however that CPPs bind with high affinity to extracellular domains of sulfated glycosaminoglycans such as heparin sulfate, heparin and others. We quantitated this interaction for a large variety of CPPs and glycosamionosulfates using high-sensitivity titration calorimetry (ITC), dynamic light scattering (DLS) and fluorescence spectroscopy. Most important was the application of this physicalchemical knowledge to living cells. We synthesized a fluorescent derivative of the HIV-1 TAT protein transduction domain and observed its uptake into non-fixated living fibroblasts with time-lapse confocal microscopy, eliminating the need of fixation. Depending on the concentration, the fluorescent CPP entered the cell within seconds. Several observations suggested that the CPP binding leads to an aggregation or “capping” of sulfated glycosaminoglycans, inducing finally endocytosis.
We further showed that the HIV-1 TAT protein transduction domain has a high affinity for double stranded DNA. The binding of this CPP leads to DNA condensation and, in parallel, a distinct reduction of fluorescence intensity is observed. This change in fluorescence quantum yield impedes the identification of uptake routes and makes the quantitative comparison of uptake efficiency by fluorescence microscopy rather difficult. As the aggregation of glycosaminoglycans on the cell surface could be the starting point of endocytosis we have studied the binding and clustering of various mono-and multivalent cell penetrating peptides and non-peptidic compounds to heparin with ITC and DLS. Finally, we were interested if antimicrobial peptides may also take advantage of sulfated glycosaminoglycans for cell entry. We investigated in detail melittin and melittin-analogs and found that melittin binds strongly to sulfated glycosamins. However, melittin appears to an exception among the amphipathic antimicrobial peptides as other peptides such as magainin 2 or nisin Z do not show such an interaction.
Lipid membranes as catalysts for protein folding
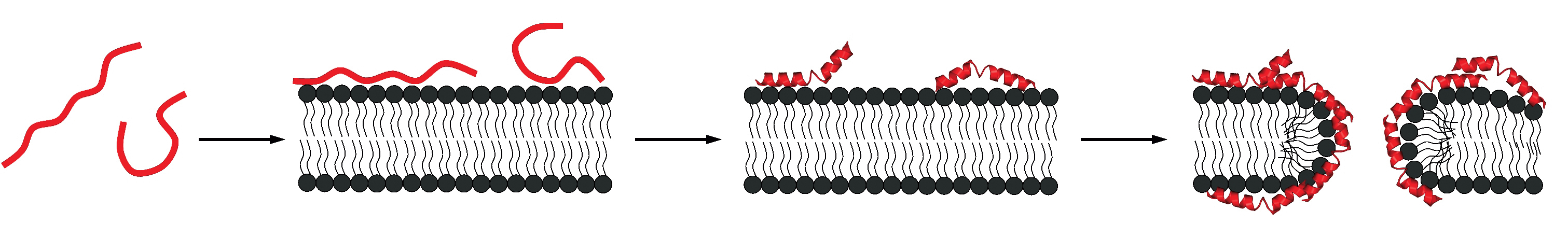
of a membrane.
Amphipathic peptides or proteins such as the bee venom melittin, the antibacterial peptide magainin 2 or the lipoprotein Apo-A1 are mainly random coil in solution but adopt an α-helical structure when bound to membranes. Likewise, a membrane-induced random coil-to-β-structure transition has been found for Alzheimer peptides such as Aβ(1-40) or fragments thereof. Melittin and related amphipathic compounds insert into the lipid membrane and modify the lipid structure. In contrast, the Aβ peptides remain on the surface of the bilayer. In a series of publications we have studied in detail the thermodynamics of the membrane-induced random-coilto- α -helix transition. This work is widely cited and the results have been confirmed by other groups.
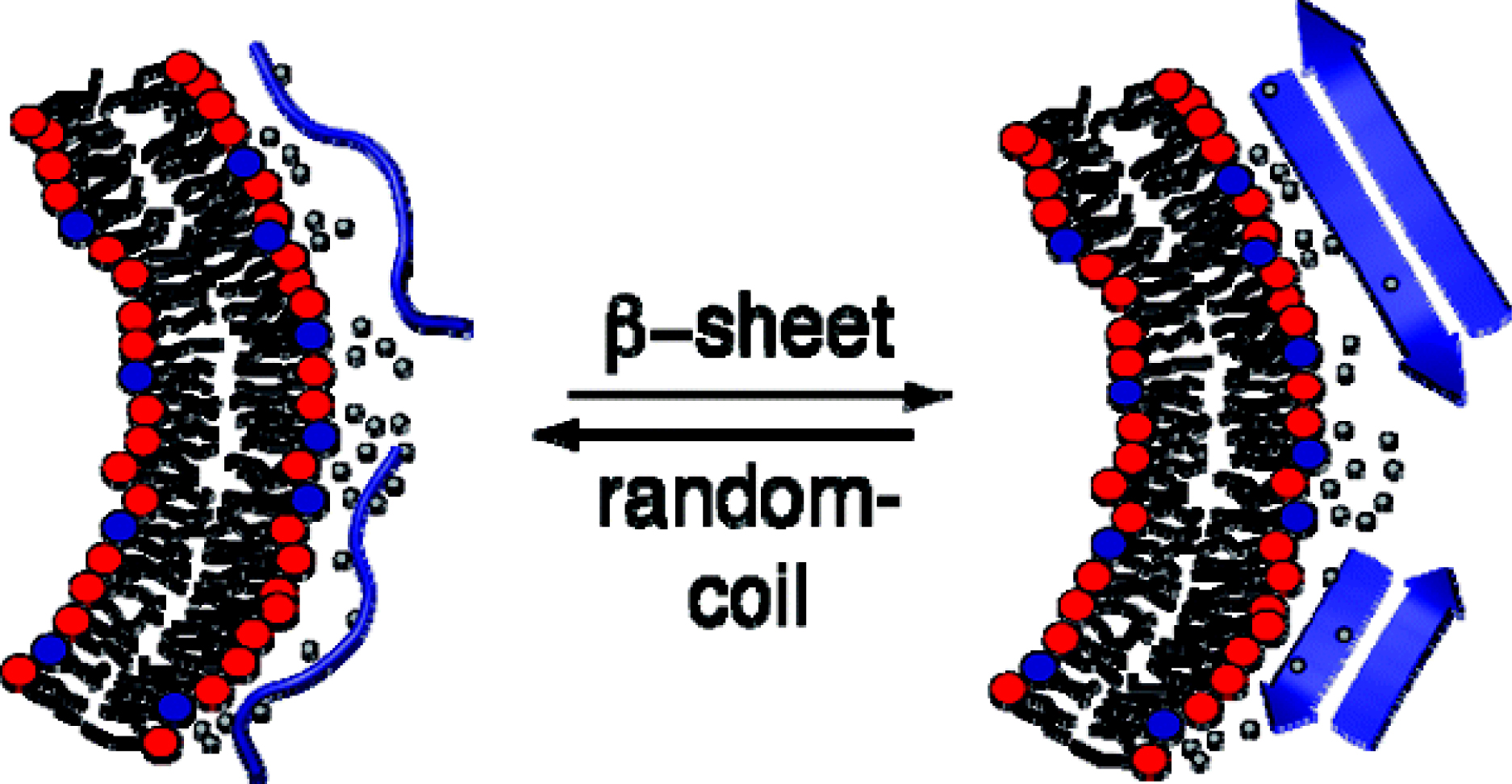
Most recently we succeeded in a related analysis for the membraneinduced random coil-to-β-structure transition. Indeed, our work appears to be the first quantitative analysis of a random coil β-structure transition. In collaboration with Y. Shai, Israel, we have applied this knowledge to a modified melittin which is β-structured on the membrane surface. The thermodynamics of this system ideally confirms the results obtained with other model systems for the rc β-structure transition.
Related projects